Lithium-Ion Batteries Explained
In today's world of portable electronics, electric vehicles, and renewable energy storage, lithium-ion batteries have become a household name. These remarkable energy storage devices have revolutionized various industries, providing high-performance and reliable power solutions. In this article, we'll delve into the fascinating world of lithium-ion batteries, exploring their composition, working principle, and widespread applications.
Understanding the Composition #
At the heart of a lithium-ion battery lies an intricate design that enables efficient energy storage and release. The key components of a typical lithium-ion battery include:
-
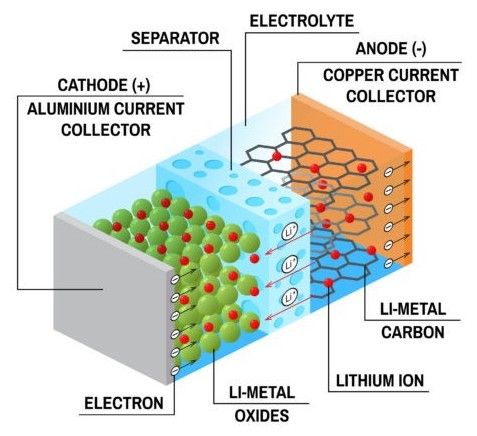
Cathode: The cathode is the positive electrode of the battery. It is typically composed of a lithium-based compound, such as lithium cobalt oxide (LiCoO2), lithium nickel manganese cobalt oxide (LiNiMnCoO2), or lithium iron phosphate (LiFePO4). The specific cathode material used determines the battery's performance characteristics.
-
Anode: The anode, the negative electrode, is commonly made of graphite. During charging, lithium ions move from the cathode to the anode, where they are stored.
-
Separator: The separator is a thin membrane placed between the cathode and anode, preventing direct contact and short circuits while allowing the flow of lithium ions.
-
Electrolyte: The electrolyte serves as a medium for lithium-ion transport between the cathode and anode. It is typically a liquid or gel containing lithium salts and organic solvents.
-
Current Collectors: The current collectors are conductive materials that facilitate the flow of electrons within the battery. They are connected to the cathode and anode, allowing for the extraction and delivery of electrical energy.
How Does It Work? #
The operation of a lithium-ion battery involves a fascinating electrochemical process. During charging, an external power source applies a voltage to the battery, causing lithium ions to migrate from the cathode to the anode through the electrolyte. Simultaneously, electrons flow through the external circuit, creating a flow of electric current that can be utilized to power various devices.
When the battery is discharged, the reverse occurs. The lithium ions move from the anode back to the cathode, releasing stored energy and powering the connected devices. This cyclic process of charging and discharging allows lithium-ion batteries to provide a reliable and rechargeable source of power.
Advantages and Applications #
Lithium-ion batteries have gained immense popularity due to their numerous advantages and wide range of applications. Here are some key benefits and areas where lithium-ion batteries excel:
-
High Energy Density: Lithium-ion batteries offer a high energy density, meaning they can store a significant amount of energy in a relatively small and lightweight package. This makes them ideal for portable electronic devices such as smartphones, laptops, and tablets, where size and weight are crucial factors.
-
Rechargeable and Long Cycle Life: Unlike disposable batteries, lithium-ion batteries are rechargeable, allowing them to be used repeatedly. They have a long cycle life, which refers to the number of charge and discharge cycles a battery can undergo before its capacity significantly degrades. This makes them a cost-effective and sustainable choice for both consumer electronics and electric vehicles.
-
High Power Output: Lithium-ion batteries can deliver high power output, making them suitable for applications that require bursts of energy, such as power tools and electric vehicles. They can provide instant power when needed, ensuring efficient operation and performance.
-
Minimal Self-Discharge: Lithium-ion batteries have a low self-discharge rate, meaning they retain their charge even when not in use. This allows devices to maintain their battery life for extended periods without the need for frequent recharging.
-
Wide Range of Applications: Lithium-ion batteries are used in various industries, including consumer electronics, electric vehicles, renewable energy storage, and even aerospace. They power our smartphones, tablets, electric cars, and contribute to the transition towards cleaner and more sustainable energy sources.
Conclusion #
Lithium-ion batteries have transformed the way we use and store energy. Their compact size, high energy density, and rechargeable nature make them an indispensable power solution for our modern world. Whether it's keeping our devices running, driving electric vehicles, or harnessing renewable energy, lithium-ion batteries continue to drive innovation and shape the future of energy storage. With ongoing research and advancements, we can expect even more exciting developments in this remarkable technology in the years to come.