What is thermal runaway?
Thermal runaway refers to a dangerous condition that can occur in batteries, where an increase in temperature leads to a self-perpetuating and uncontrollable rise in temperature.
When a battery undergoes thermal runaway, it experiences an internal heat generation that surpasses its ability to dissipate heat, causing a positive feedback loop. The heat generated within the battery accelerates the chemical reactions occurring inside, which further increases the temperature. This cycle continues until the battery becomes unstable, potentially leading to overheating, venting, or in extreme cases, explosion or fire.
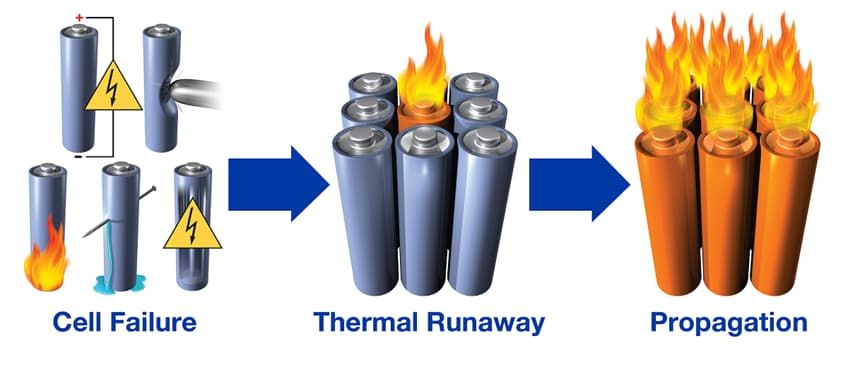
Several factors can contribute to thermal runaway in batteries, including:
Overcharging #
Overcharging a battery can cause the release of excess energy, leading to an increase in temperature. If the battery management system fails to prevent overcharging or if the charging process is not properly regulated, it can result in thermal runaway.
Overheating #
External factors such as exposure to high temperatures, direct sunlight, or placing the battery near a heat source can raise the temperature of the battery. If the battery's internal temperature reaches a critical threshold, it can trigger thermal runaway.
Physical damage #
Any physical damage to the battery, such as punctures, deformations, or short circuits, can disrupt the internal structure and lead to the release of energy. This can initiate thermal runaway and result in a hazardous situation.
Manufacturing defects #
Poorly manufactured batteries or cells with defects in their design or construction may have a higher likelihood of experiencing thermal runaway. Manufacturing defects can compromise the integrity of the battery, making it more susceptible to thermal runaway.
Battery chemistries most susceptible to thermal runaway #
While thermal runaway can occur in any battery chemistry under certain conditions, some battery chemistries are considered more susceptible to this phenomenon than others. The following battery chemistries are known to have a higher risk of thermal runaway:
Lithium Cobalt Oxide (LCO) #
LCO batteries have a high energy density, making them commonly used in portable electronics like smartphones and laptops. However, they are relatively more prone to thermal runaway compared to other lithium-ion chemistries. LCO batteries contain cobalt, which can undergo exothermic reactions and release oxygen at high temperatures, contributing to thermal instability.
Lithium Nickel Cobalt Aluminum Oxide (NCA) #
NCA batteries, often used in electric vehicles, provide a high energy density and excellent power output. However, they have a reduced tolerance for high temperatures and are considered more susceptible to thermal runaway. NCA batteries contain cobalt and nickel, which can contribute to thermal instability when exposed to extreme conditions.
Lithium Manganese Oxide (LMO) #
LMO batteries offer a good compromise between energy density, power output, and safety. They are commonly used in power tools and medical devices. While LMO batteries exhibit better thermal stability compared to LCO and NCA chemistries, they can still be susceptible to thermal runaway if subjected to severe abuse conditions.
Safety measures to improve thermal stability #
Preventing and mitigating thermal runaway is crucial for the safe operation of batteries. Battery management systems (BMS) are designed to monitor and control various parameters, including temperature, voltage, and current, to prevent overcharging and overheating. Thermal management systems, such as cooling mechanisms or heat dissipation techniques, are also employed to regulate the temperature of the battery.
Other battery chemistries, such as Lithium Iron Phosphate (LFP) and Lithium Titanate (LTO), are known for their enhanced thermal stability. These chemistries have a lower risk of thermal runaway and are widely used in applications where safety and long cycle life are crucial, such as residential energy storage and electric grid applications.
Advancements in battery technology and safety measures have significantly improved the thermal stability of these chemistries over the years. Manufacturers employ various techniques to enhance the safety of lithium-ion batteries, such as incorporating thermal management systems, improving cell design, and utilizing advanced materials.
In summary, thermal runaway is a critical concern in battery systems, and precautions must be taken to prevent it. By implementing appropriate safety measures, monitoring systems, and adhering to proper handling and usage guidelines, the risk of thermal runaway can be significantly minimized, ensuring the safe and reliable operation of batteries.